Chondroitinase AC & Chondroitinase B
Using high purity enzymes will improve the quality of your results.
The chondroitinases are available in liquid form.
IBEX produces a family of high purity recombinant glycosaminoglycan (GAG) lyases; among them Chondroitinase AC and Chondroitinase B (as well as Heparinase I, Heparinase II, Heparinase III).
The IBEX chondroitinases are widely used in research and as quality control reagents.
IBEX has developed a proprietary Flavobacterium heparinum expression system which produces glycosylated enzymes exactly the same as GAGS produced from the native strain, but with less cross-contaminating GAG enzymes
IBEX chondroitinases do not contain external proteins such as bovine serum albumin (BSA), nor preservatives.
| ENZYME | SUBSTRATE |
| Chondroitinase AC(EC 4.2.2.5) Eliminative degradation of polysaccharides containing 1,4-b-D-hexosaminyl and 1,3-b-D-glucuronosyl linkages to disaccharides containing 4-deoxy-b-D-gluc-4-enuronosyl groups. Chondroitinase AC cleaves chondroitin sulfates A and C. | 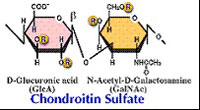 |
| Chondroitinase B (EC 4.2.2.19) Chondroitinase B cleaves dermatan sulfate. | 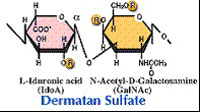 |


